Research Interests
Nucleophilic Organocatalysis
Nucleophilic catalysts are of outstanding importance in synthetic protocols for the synthesis of ethers, esters and amides. Using a combination of experimental and theoretical techniques we develop new catalysts of enhanced reactivity or selectivity. These projects include (a) the synthesis of new catalysts, mostly based on the pyridine or triarylphosphane motif; (b) kinetic studies for the characterization of catalyst efficiency (NMR and GC); (c) theoretical studies of the reaction mechanism and of conformational properties of the reaction components. This tight interplay between experiment and theory yields a better understanding of the true source of catalytic efficiency.
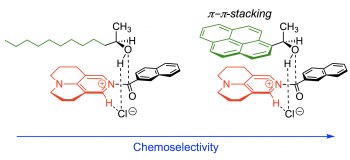
10) J. Helberg, M. Marin-Luna, H. Zipse,
"Cheoselectivity in Esterification Reactions - Size Matters After All"
Synthesis 2017,49, 3460 - 3470. [DOI: 10.1055/s-0036-1588854]
9) P. Patschinski, H. Zipse,
"Leaving Group Effects on the Selectivity of the Silylation of Alcohols: The Reactivity–Selectivity Principle Revisited"
Org. Lett. 2015,17, 3318 - 3321. [DOI: 10.1021/acs.orglett.5b01536]
8) P. Patschinski, C. Zhang, H. Zipse,
"The Lewis Base-Catalyzed Silylation of Alcohols — A Mechanistic Analysis"
J. Org. Chem. 2014, 79, 8348 – 8357. [DOI: 10.1021/jo5016568]
7) C. Lindner, Y. Liu, K. Karaghiosoff, B. Maryasin, H. Zipse,
"The Aza-Morita–Baylis–Hillman Reaction: A Mechanistic and Kinetic Study"
Chem. Eur. J. 2013, 19, 6429 - 6434. [DOI: 10.1002/chem.201204006]
6) E. Larionov, F. Achrainer, J. Humin, H. Zipse,
"The Catalytic Potential of Substituted Pyridines in Acylation Reactions: Theoretical Prediction and Experimental Validation"
ChemCatChem 2012, 4, 559 - 566. [DOI: 10.1002/cctc.201100313]
5) E. Larionov, M. Mahesh, A. C. Spivey, H. Zipse,
"Theoretical Prediction of Selectivity in Kinetic Resolution of Secondary Alkcohols Catalyzed by Chiral DMAP Derivatives"
J. Am. Chem. Soc. 2012, 134, 9390 - 9399. [DOI: 10.1021/ja302420g]
4) V. D'Elia, Y. Liu, H. Zipse,
"Immobilized DMAP-Derivatives Rivaling Homogeneous DMAP"
Eur. J. Org. Chem. 2011, 1527 - 1533. [DOI: 10.1002/ejoc.201001507]
3) C. B. Fischer, S. Xu, H. Zipse,
"Steric Effects in the Uncatalyzed and DMAP-Catalyzed Acylation of Alcohols - Quantifying the Window of Opportunity in Kinetic Resolution Experiments"
Chem. Eur. J. 2006, 12, 5779 - 5784. [DOI: 10.1002/chem.200600280]
2) S. Xu, I. Held, B. Kempf, H. Mayr, W. Steglich, H. Zipse,
"The DMAP-Catalyzed Acetylation of Alcohols - A Mechanistic Study (DMAP = 4-(Dimethylamino)pyridine)"
Chem. Eur. J. 2005, 11, 4751 - 4757. [DOI: 10.1002/chem.200500398]
1) M. R. Heinrich, H. S. Klisa, H. Mayr, W. Steglich, H. Zipse,
"Enhancing the Catalytic Activity of 4-(Dialkylamino)pyridines by Conformational Fixation"
Angew. Chem. 2003, 115, 4975 - 4977; Angew. Chem. Int. Ed. 2003, 42, 4826 - 4828. [DOI: 10.1002/anie.200352289]
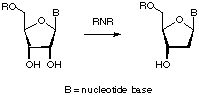
Theoretical studies of radicals in enzymatic catalysis
What type of radical chemistry is used in enzymatic catalysis? This is the central question we try to answer in our model studies of enzymes that are known to use radical chemistry during substrate conversion such as ribonucleotide reductases (RNRs) or pyruvate formate lyase (PFL). Of particular interest in these projects is the question how well known radical reactions such as hydrogen atom abstraction are influenced by the hydrogen bonding network present in the enzyme active site.
1) D. M. Smith, W. Buckel, H. Zipse
"Deprotonation of Enoxy Radicals: Theoretical Validation of a 50-Year-Old Mechanistic Proposal"
Angew. Chem. 2003, 115, 1911 - 1915; Angew. Chem. Int. Ed. 2003, 42, 1867 - 1870.
2) H. Zipse
"The Influence of Hydrogen Bonding Interactions on the C-H Bond Activation Step in Class 1 Ribonucleotide Reductases"
Org. Biomol. Chem. 2003, 1, 692 - 699.
3) K. Condic-Jurkic, V. T. Perchyonok, H. Zipse, D. M. Smith
"On the modeling of arginine-bound carboxylates: A case study with Pyruvate Formate-Lyase"
J. Comp. Chem. 2008, 29, 2425 - 2433.
4) H. Zipse, E. Artin, S. Wnuk, G. J. S. Lohman, D. Martino, R. G. Griffin, S. Kacprzak, M. Kaupp, B. Hoffman, M. Bennati, J. Stubbe, N. Lees,
"Structure of the Nucleotide Radical Formed during Reaction of CDP/TTP with the E441Q-.alpha.2.beta.2 of E. coli Ribonucleotide Reductase"
J. Am. Chem. Soc. 2009, 131, 200 - 211.
5) K. Condic-Jurkic, H. Zipse, D. M. Smith
"A Compound QM/MM Procedure: Comparative Performance on a Pyruvate Formate-Lyase Model System"
J. Comp. Chem. 2010, 31, 1024 - 1035.
6) J. Hioe, H. Zipse
"Radicals in enzymatic catalysis - a thermodynamic perspective"
Faraday Disc. 2010, 145, 301 - 313 (original manuscript).
Faraday Disc. 2010, 145, 381 - 409 (general discussion I).
7) J. Hioe, G. Savasci, H. Brandt, H. Zipse
"The Stability of C(alpha) Peptide Radicals - why Glycyl Radical Enzymes?"
Chem. Eur. J. 2011, 17, 3781 - 3789.
8) K. Condic-Jurkic, A. Smith, H. Zipse, D. M. Smith
"The Protonation States of the Active-Site Histidines in (6-4) Photolyase"
J. Chem. Theory Comput. 2012, 8, 1078 - 1091.
9) J. Hioe, H. Zipse
"Hydrogen Transfer in SAM-Mediated Enzymatic Radical Reactions"
Chem. Eur. J. 2012, 18, 16463 - 16472.
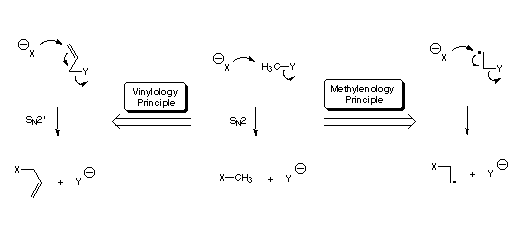
Theoretical studies of the interplay between radical and ionic reactions
How do radicals influence the course of ionic reactions? What happens to the SN2 substitution mechanism if we introduce a neighboring radical center? Does a "Methylenology-Principle" exist, which relates nucleophilic substitution reactions in radicals to those in closed shell substrates? Are there analogies between addition reactions to C-C double bonds (such as in the Michael- or SN2'-reactions) and addition reactions to radical centers? These are just some of the questions we try to answer using modern ab initio methods in combination with various approaches to model solvent effects. The aim of these studies is to improve the understanding of a large number of experimental observations on ionic reactions in radicals.
1) Y. Wang, S. Grimme, H. Zipse,
"Charge Separation and Charge Distribution in Rearrangement Reactions of ß-(Phosphatoxyx)alkyl Radicals"
J. Phys. Chem. A 2004, 108, 2324 - 2331.
2) H. Zipse,
"Charge distribution and charge separation in radical rearrangement reactions"
Adv. Phys. Org. Chem. 2003, 38, 111 - 130.
Theoretical and experimental investigation of the chemistry of radical cations
What happens to alkene radical cations in water? For several years we have been trying to answer this seemingly trivial question using a variety of theoretical methods. Ab initio studies of simple model systems such as ethylene radical cations reacting with a single water molecule convinced us that solvent effects will be exceedingly important in the reaction mechanism. Reactive or unreactive solvation? The answer strongly depends on the way solvent effects are described theoretically!
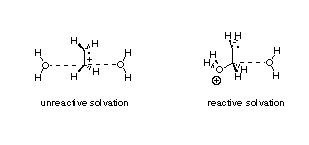
1) M. Mohr, H. Zipse
"Does the cationic or the radical character dominate the reactivity of alkene radical cations towards solvent molecules?"
Phys. Chem. Chem. Phys. 2001, 3,1246 - 1252.