Mulliken Population Analysis
Directly following the output of molecular orbital information Gaussian prints a Mulliken population analysis. A full Mulliken population analysis is obtained using pop=full. In this case the molecular orbital information is followed by the current density matrix D (a n*n matrix with n = number of basis functions). The matrix elements Di,j are calculated as the sum of products okci,kcj,k over all k molecular orbitals. The occupation numbers ok for single determinant wavefunctions are either 0 (virtual orbital), 1 (singly occupied orbitals), or 2 (fully occupied orbital), and the molecular orbital coefficient ci,k is that for basis function i in molecular orbital k. For formaldehyde (CH2O, C2v symmetry) the following results are obtained at the HF/STO-3G level of theory:
DENSITY MATRIX.
1 2 3 4 5
1 1 C 1S 2.07173
2 2S -0.22403 0.84547
3 2PX 0.00000 0.00000 0.74263
4 2PY 0.00000 0.00000 0.00000 0.63487
5 2PZ 0.01681 -0.07821 0.00000 0.00000 0.55273
6 2 O 1S 0.01218 -0.00171 0.00000 0.00000 -0.04249
7 2S -0.00751 -0.17656 0.00000 0.00000 -0.01027
8 2PX 0.00000 0.00000 0.82369 0.00000 0.00000
9 2PY 0.00000 0.00000 0.00000 0.15487 0.00000
10 2PZ 0.15078 -0.42873 0.00000 0.00000 -0.58493
11 3 H 1S -0.10842 0.28876 0.00000 0.45096 -0.25196
12 4 H 1S -0.10842 0.28876 0.00000 -0.45096 -0.25196
6 7 8 9 10
6 2 O 1S 2.11060
7 2S -0.46427 2.05057
8 2PX 0.00000 0.00000 0.91359
9 2PY 0.00000 0.00000 0.00000 1.90476
10 2PZ -0.09605 0.55492 0.00000 0.00000 1.02854
11 3 H 1S 0.00892 -0.01948 0.00000 -0.35936 0.11723
12 4 H 1S 0.00892 -0.01948 0.00000 0.35936 0.11723
11 12
11 3 H 1S 0.63094
12 4 H 1S -0.24573 0.63094
|
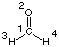 |
As always these results are given with the system in its "Standard Orientation". The first step in the "Full Mulliken population analysis" involves multiplication of the density matrix elements Di,j with the corresponding overlap matrix elements Si,j (again a n*n matrix). In case a basis of normalized, non-orthogonal basis functions is used, the matrix elements Si,i equate to unity. This implies that the diagonal elements of the D- and DS-matrices are identical:
Full Mulliken population analysis:
1 2 3 4 5
1 1 C 1S 2.07173
2 2S -0.05564 0.84547
3 2PX 0.00000 0.00000 0.74263
4 2PY 0.00000 0.00000 0.00000 0.63487
5 2PZ 0.00000 0.00000 0.00000 0.00000 0.55273
6 2 O 1S 0.00000 -0.00006 0.00000 0.00000 -0.00260
7 2S -0.00027 -0.06377 0.00000 0.00000 -0.00452
8 2PX 0.00000 0.00000 0.17189 0.00000 0.00000
9 2PY 0.00000 0.00000 0.00000 0.03232 0.00000
10 2PZ -0.00906 0.13729 0.00000 0.00000 0.18336
11 3 H 1S -0.00658 0.13982 0.00000 0.17576 0.06313
12 4 H 1S -0.00658 0.13982 0.00000 0.17576 0.06313
6 7 8 9 10
6 2 O 1S 2.11060
7 2S -0.10990 2.05057
8 2PX 0.00000 0.00000 0.91359
9 2PY 0.00000 0.00000 0.00000 1.90476
10 2PZ 0.00000 0.00000 0.00000 0.00000 1.02854
11 3 H 1S 0.00004 -0.00137 0.00000 -0.01289 -0.00823
12 4 H 1S 0.00004 -0.00137 0.00000 -0.01289 -0.00823
11 12
11 3 H 1S 0.63094
12 4 H 1S -0.03712 0.63094
The example used here illustrates two problems that come up when the DS-matrix elements are interpreted as atomic orbital occupation numbers. The first problem consists in occupation numbers larger than 2.0, in clear violation of the Pauli exclusion principle. Second, negative occupation numbers are clearly unphysical and cannot be interpreted in a classical sense. The interpretation of single DS-matrix elements is therefore not very meaningful.
Summation of diagonal elements of the DS-matrix together with one half of all corresponding off-diagonal elements gives the gross orbital populations for each of the contributing atomic basis functions. That the off-diagonal elements can be distributed equally among the contributing atomic centers is one of the central assumptions made in the Mulliken population analysis.
Gross orbital populations:
1
1 1 C 1S 1.99361
2 2S 1.14294
3 2PX 0.91452
4 2PY 1.01871
5 2PZ 0.85523
6 2 O 1S 1.99812
7 2S 1.86937
8 2PX 1.08548
9 2PY 1.91130
10 2PZ 1.32368
11 3 H 1S 0.94352
12 4 H 1S 0.94352
Alternatively, the diagonal and off-diagonal elements can also be summed up for each of the atoms:
Condensed to atoms (all electrons):
1 2 3 4
1 C 4.736157 0.444580 0.372141 0.372141
2 O 0.444580 7.788268 -0.022449 -0.022449
3 H 0.372141 -0.022449 0.630941 -0.037118
4 H 0.372141 -0.022449 -0.037118 0.630941
Summing up the contributions for each atom and subtracting the nuclear charges gives the atomic charges (either separately for each atom or with the hydrogen charges summed up with the adjacent non-hydrogen atoms.
Total atomic charges:
1
1 C 0.074981
2 O -0.187950
3 H 0.056484
4 H 0.056484
Sum of Mulliken charges= 0.00000
Atomic charges with hydrogens summed into heavy atoms:
1
1 C 0.187950
2 O -0.187950
3 H 0.000000
4 H 0.000000
Sum of Mulliken charges= 0.00000
|
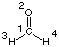 |
Known problems of the Mulliken population analysis include large changes of the computed atomic charges with small changes in the underlying basis sets and the overestimation of the covalent character of a bond. These deficiencies can nicely be demonstrated using lithium fluoride as an example. The following table lists the lithium charge in lithium fluoride as calculated with the Mulliken population analysis and a variety of Pople basis sets. The Becke3LYP/6-31G(d) optimized geometry with r(Li-F)=155.139 pm has been used for this purpose.
|
Li - F |
While it is comforting to see that the lithium charge is predicted to be positive with all methods used here, the variation as a function of basis set choice is rather large (between 0.078 and 0.675 at the Becke3LYP level of theory). There are, of course, also variations due to changing the Hamiltonian (here from RHF to RB3LYP), but these appear to be rather constant and comparatively small. It can also be seen that, even with the largest Pople basis sets used here, the degree of charge separation between Li and F is underestimated with the Mulliken population analysis. The Natural Population Analysis (NPA), in contrast, predicts a much more positive charge on Li.
A detailed overview of the effects of the basis set and the Hamiltonian on the charge distribution in water can be found in: F. Martin, H. Zipse, J. Comp. Chem. 2005, 26, 97 - 105. A copy of the pdf file is available here.