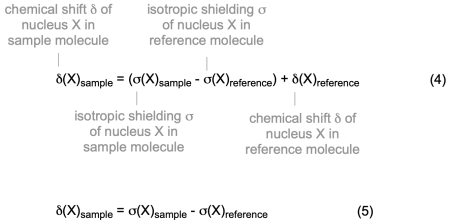
8.2 Conversion of Isotropic Shieldings to Chemical Shifts
The most commonly used NMR active nuclei in solution phase chemistry are those with nuclear spin quantum number I = 1/2 collected in Table 8.2.1.[1,2] Of particular importance for our purposes here are the reference compounds selected historically for defining the zero of NMR chemical shift scales for each nucleus. Tetramethylsilane (TMS) features prominently as the zero reference for 1H, 13C, and 29Si in organic solvents. For solubility reasons this is replaced by the sodium salt of 3-(trimethylsilyl)propanesulfonate (DSS) for measurements in D2O with particular relevance in biomolecular NMR. TMS and DSS are used as internal references in the sense that these compounds are added to the analyte solution. This is not easily possible with other references as, for example, with 85% phosphoric acid in water. Most commonly, this latter compound is used as an external reference added in a closed capilary to the NMR tube holding the analyte solution.
Table 8.2.1. Important NMR active nuclei with I = 1/2.
| Nuclei | Natural abundance |
Reference compound |
Alternative reference |
| 1H | 99.99 | Me4Si (TMS) | Sodium-3-(trimethylsilyl)propanesulfonate (DSS, in D2O) |
| 13C | 1.07 | Me4Si (TMS) | Sodium-3-(trimethylsilyl)propanesulfonate (DSS, in D2O) |
| 15N | 0.37 | MeNO2 | - |
| 19F | 100.00 | FCCl3 | - |
| 29Si | 4.6832 | Me4Si (TMS) | - |
| 31P | 100.00 | 85% H3PO4 | - |
As described by eq. (4), chemical shift values measured in solution depend on isotropic shielding values in a (nearly) linear fashion. In case the reference compound chosen for shielding calculations equates to the respective reference compound defining the zero of the chemical shift scale, eq. (4) simplifies to eq. (5) as the chemical shift value of nucleus X in the reference compound equates to zero. This can easily be demonstrated using the CHCl3 solvent signal observed in many 1H NMR measurements in CDCl3 solution at +7.26 ppm. According to eq. (5), all we need to predict this value are the 1H shielding values in CHCl3 and TMS as summarized in Figure 8.2.1.
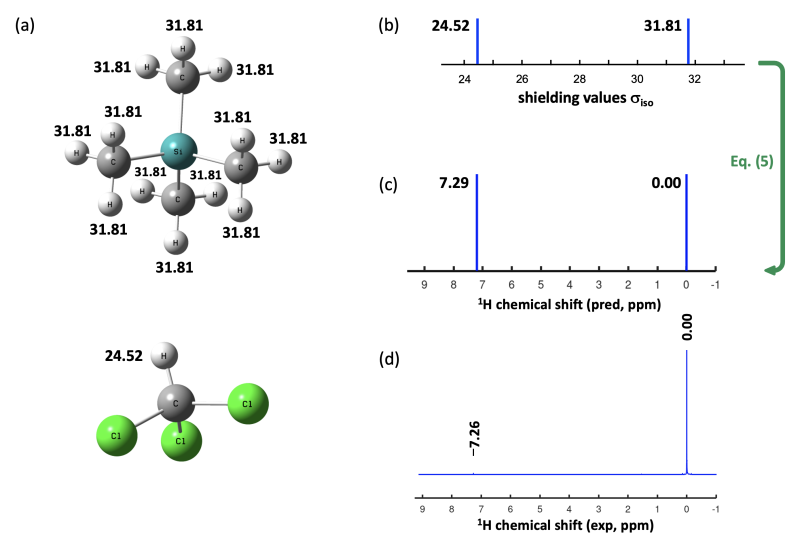
Figure 8.2.1. 1H isotropic shieldings for CHCl3 and TMS calculated at M06-2X/def2-TZVPP level of theory
with Gaussian presented (a) numerically, and (b) graphically. (c) 1H Chemical shifts calculated from shielding
values. (d) Experimental 1H NMR spectrum of TMS in CDCl3, where a small CHCl3 solvent signal is visible.
The gas phase 1H shielding values calculated with Gaussian at M06-2X/def2-TZVPP level amount to 24.52 ppm for CHCl3 and 31.81 for TMS. Applying eq. (5) predicts the CHCl3 signal at +7.29 ppm, which is in impressively good agreement with the measurements in CDCl3 solution at +7.26 ppm. Using the gas phase 1H shielding values calculated at the same level with ORCA of 24.23 ppm for CHCl3 and 31.49 for TMS yields a chemical shift for CHCl3 of +7.26 ppm, again in seemingly quantitative agreement with experiment. Whatever makes the gas phase 1H shielding values calculated at M06-2X/def2-TZVPP level in both programs different by a quarter of a ppm seems to be systematic enough to cancel when chemical shift values are calculated with eq. (5).
Eqs. (4) and (5) can also employed for the prediction of 13C NMR chemical shifts as can be illustrated with the 13C NMR solvent signal of CDCl3. As shown in Figure 8.2.2d, this signal appears as a 1:1:1 triplet (due to coupling of 13C (I = 1/2) with the attached deuterium atom 2H (I = 1)) at +77.01 ppm. The shielding calculations will not reflect the coupling of NMR-active nuclei, so all we will do is predict the central position of this triplet signal. 13C shielding calculations at the M06-2X/def2-TZVPP level of theory with Gaussian predict shielding values of 85.91 ppm for CHCl3 and 187.50 ppm for TMS. Applying eq. (5) predicts the solvent signal to be located at +101.68 ppm, which is significantly different from the experimentally observed value at +77.01 ppm (Figure 8.2.2).
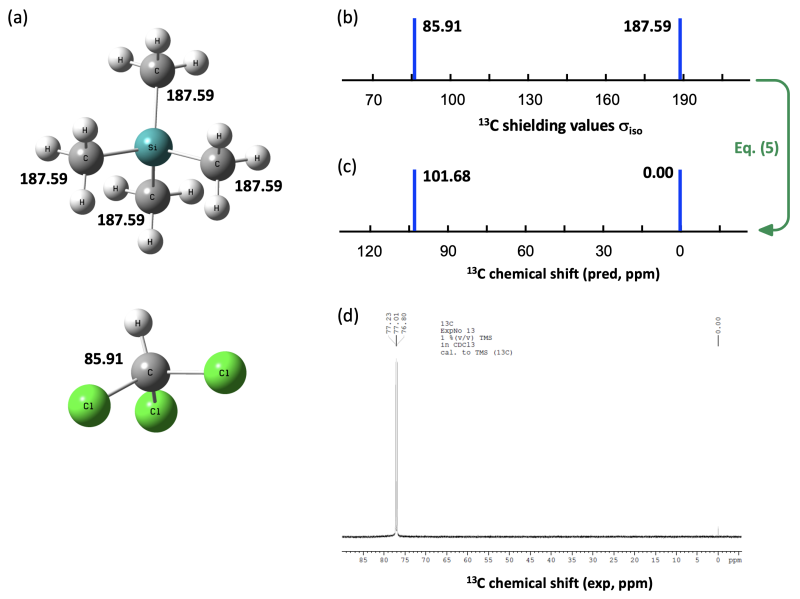
Figure 8.2.2. 13C Isotropic shielding values in TMS and CHCl3 calculated at M06-2X/def2-TZVPP level of theory
with Gaussian presented (a) numerically, and (b) graphically. (c) 13C Chemical shifts calculated from shielding values.
(d) Experimental 13C NMR spectrum of TMS in CDCl3, where the CDCl3 signal appears as a triplet at +77.01 ppm.
Possible reasons for deviations between experimentally measured and theoretically predicted NMR chemical shifts include: (a) inappropriate quantum mechanical methods; (b) inappropriate basis set choice; (c) solvent effects; and (d) inappropriate choice of reference systems.
References
[1] (a) R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, R. Goodfellow, P. Granger, “NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001)”, Pure Appl. Chem. 2001,73,1795−1818. (b) R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, P. Granger, R. E. Hoffman, K. W. Zilm,“Further Conventions for NMR Shielding and Chemical Shifts (IUPAC Recommendations 2008)”, Pure Appl. Chem. 2008, 80, 59−84.
[2] G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw, K. I. Goldberg, “NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist”, Organometallics 2010, 29, 2176−2179.
last changes: 11.11.2024, Imon Mandal and Hendrik Zipse, supported by the RTG 2620 on "Ion Pair Effects in Molecular Reactivity" questions & comments to: zipse@cup.uni-muenchen.de